Inherited disorders tied to DNA repair, genomic imprinting, and dynamic (triplet-repeat) mutations appear on Step 1 as pattern-recognition problems that reward mechanistic thinking. Stems typically describe age at presentation, a classic trigger (UV exposure, chemotherapy sensitivity, paternal vs maternal inheritance), a sentinel tumor, or a stereotyped neurologic or developmental phenotype. The fastest way to score these questions is to map the clinical signal to a repair pathway or parent-of-origin rule first, then to the prototype gene and disease. For example, “freckling and early skin cancers after sun exposure” points to nucleotide excision repair failure (xeroderma pigmentosum), while “MSI-high colon cancer in a 45-year-old with multiple relatives” signals mismatch repair deficiency (Lynch syndrome). The second strategic layer is phase-of-cell-cycle anchoring. High-yield associations include: mismatch repair policing replication errors during S phase; nucleotide excision repair clearing bulky adducts in G1; base excision repair continuously fixing small non-helix-distorting lesions; and double-strand break repair (homologous recombination, non-homologous end joining) guarding genome integrity when both strands are severed. When a stem mentions hypersensitivity to ionizing radiation or crosslinking agents, jump to double-strand break and interstrand crosslink pathways (ATM/ATR, BRCA1/2, Fanconi). Parent-of-origin phenomena are framed with deletions or uniparental disomy on specific loci. Two imprinted syndromes dominate: Prader–Willi (loss of paternal expression on 15q11-q13) and Angelman (loss of maternal UBE3A on 15q11-q13). Stems often emphasize neonatal hypotonia with hyperphagia and later obesity (Prader–Willi) versus ataxia, seizures, and inappropriate laughter (Angelman). Test writers also probe Beckwith–Wiedemann (11p15 dysregulation) through macroglossia, hemihyperplasia, and embryonal tumors. Finally, triplet-repeat conditions prize two cues: anticipation and the specific trinucleotide. CGG (Fragile X), CAG (Huntington), CTG (myotonic dystrophy type 1), and GAA (Friedreich ataxia) are the indispensable quartet. Parent-of-origin effects matter: Huntington expansions classically track with paternal transmission; Fragile X expansions occur during oogenesis. Build flash pathways: “CGG → hypermethylation of FMR1 → intellectual disability with long face and macroorchidism,” or “CAG → toxic gain-of-function huntingtin → caudate atrophy and chorea.” The exam reward is speed through confident linkage of phenotype → mechanism → gene. 1) Orientation & Exam Strategy: What Step 1 Really Tests Here
Effective recall starts with a compact map linking lesion type to the corrective machinery and a sentinel clinical phenotype. Think in four bins: (1) removal of bulky adducts that distort the helix, (2) excision of small, non-bulky base damage, (3) post-replicative mismatch correction, and (4) rescue from double-strand breaks and crosslinks. Note that checkpoint kinases (ATM/ATR) orchestrate damage responses and recruiting; their dysfunction amplifies radiosensitivity and malignancy risk. Bloom syndrome (BLM helicase) exemplifies excess sister-chromatid exchange and broad cancer risk, sitting at the interface of replication and recombination control. Fanconi anemia, a genetically heterogeneous failure of interstrand crosslink repair (FANC gene network), yields bone-marrow failure, congenital anomalies, and hypersensitivity to crosslinking agents. These exemplars let you predict stem details: “aplastic anemia after DNA crosslinking chemotherapy” is a Fanconi clue, while “sunburns leading to early skin cancers” is XP. 2) DNA Repair Panorama: Pathways, Lesions, and Prototypical Diseases
Pathway Canonical Lesion Key Players Prototype Disorders (Board Signals) Nucleotide Excision Repair (NER) Bulky adducts; UV-induced thymine dimers XPA–XPG endonucleases; DNA pol δ/ε; ligase Xeroderma pigmentosum → photosensitivity, early BCC/SCC/melanoma Base Excision Repair (BER) Oxidation, alkylation, deamination; abasic sites DNA glycosylases, AP endonuclease, DNA pol β MUTYH-associated polyposis; scattered links to neurodegeneration Mismatch Repair (MMR) Replication slippage; small insertion/deletion loops MSH2/MSH6, MLH1/PMS2 Lynch syndrome (HNPCC) ± Muir–Torre (sebaceous tumors) Homologous Recombination (HR) Double-strand breaks; collapsed forks BRCA1/2, RAD51, FANCD2/I; ATM/ATR checkpoint BRCA-related breast/ovarian cancer; Fanconi anemia; ataxia-telangiectasia features Non-Homologous End Joining (NHEJ) Double-strand breaks (error-prone ligation) Ku70/80, DNA-PKcs, Ligase IV, XRCC4 Ligase IV deficiency; radiosensitive SCID-like phenotypes
Nucleotide excision repair (NER) removes bulky lesions that distort the double helix, classically UV-induced cyclobutane pyrimidine dimers. Damage recognition proteins recruit endonucleases (XPA–XPG) that incise on both sides of the lesion, excise an oligonucleotide patch, and fill the gap with high-fidelity polymerases and ligase. Loss-of-function variants in these XP genes cause xeroderma pigmentosum. Clinical hallmarks include severe sunburns in childhood, lentiginous freckling on sun-exposed skin, ocular photophobia, and early onset of basal cell, squamous cell, and melanoma skin cancers. The unifying mechanism is unchecked mutagenesis after UV exposure due to failed lesion removal. Base excision repair (BER) handles small, non-bulky lesions such as oxidized, alkylated, or deaminated bases. A lesion-specific DNA glycosylase removes the damaged base, creating an apurinic/apyrimidinic (AP) site. AP endonuclease cleaves the backbone, DNA polymerase β inserts the correct nucleotide, and DNA ligase seals the nick. The canonical Step-1 vignette ties nitrite-mediated deamination (e.g., cytosine → uracil) or oxidative damage to BER. While most BER defects are embryonically lethal, a clinically testable entity is MUTYH-associated polyposis, caused by biallelic MUTYH glycosylase mutations, leading to adenomatous polyps and colorectal cancer risk resembling attenuated APC disease. Distinguishing features on exams center on lesion type and exposure. “Sun-sensitive child with multiple skin cancers” → NER failure (XP). “Diffuse adenomatous polyposis with biallelic glycosylase mutation” → BER defect (MUTYH). Timing also helps: NER is prominent in G1 as the cell surveys for environmental insults before S phase, whereas BER runs constitutively to mop up metabolic base damage. A typical distractor is confusing NER with photolyase-mediated direct reversal (present in many organisms but not humans); Step 1 expects NER as the human solution for UV dimers. Practical tie-ins include photosensitivity counseling in XP and cascade testing for families. Molecular confirmation generally employs targeted sequencing of XP genes for NER disorders and panel testing that includes MUTYH for BER-related polyposis. The key learning pearl remains the bulky vs small lesion dichotomy: once that is recognized, remaining steps—endonuclease excision versus glycosylase incision—fall into place quickly under time pressure. Practice exactly how you’ll be tested—adaptive QBank, live CCS, and clarity from your data.3) Nucleotide vs Base Excision Repair: UV Dimers, Deaminations, and Clinical Correlates
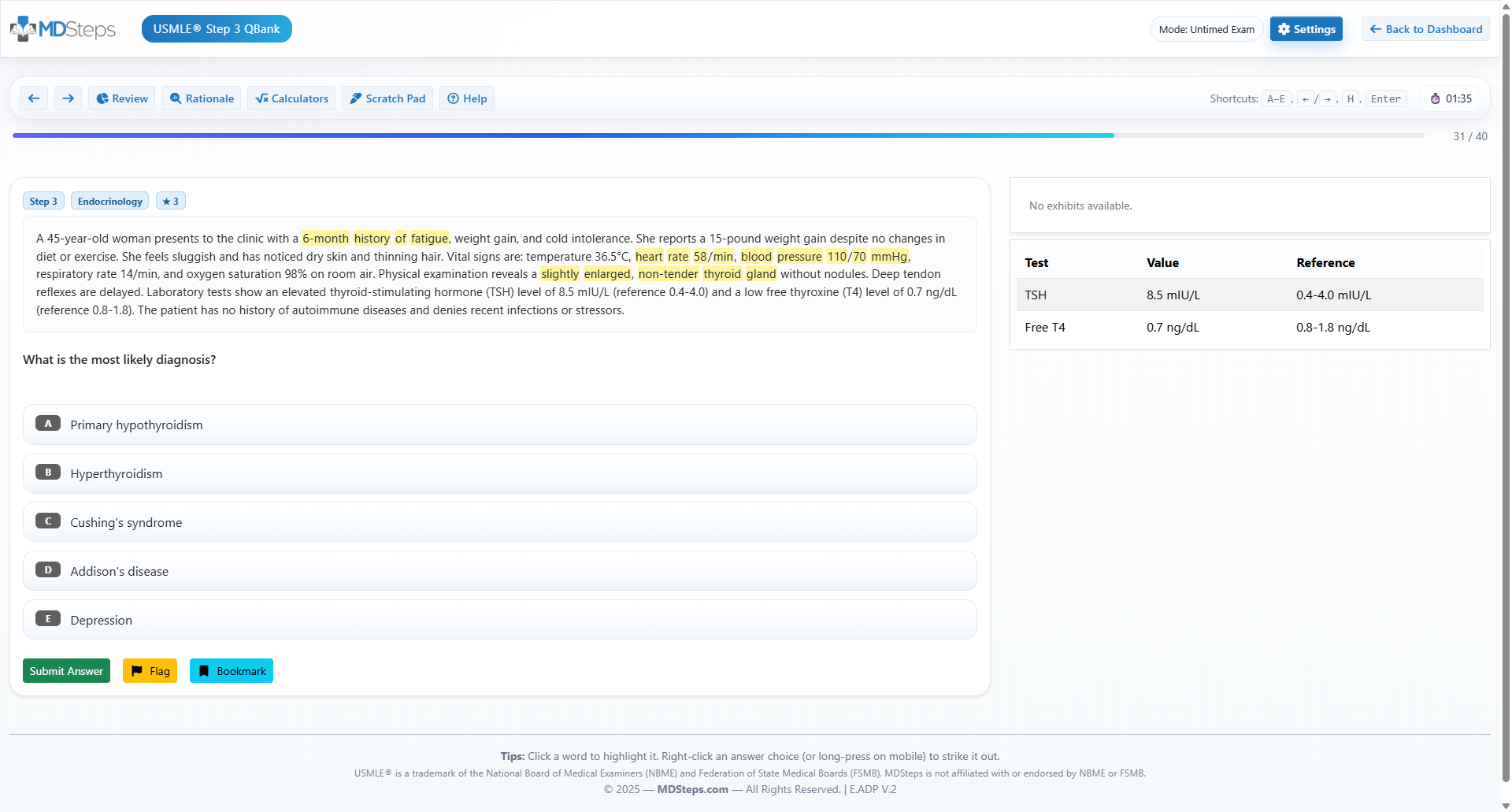
Master your USMLE prep with MDSteps.
100+ new students last month.
Mismatch repair (MMR) corrects replication errors that escape the polymerase proofreader—single-base mismatches and short insertion/deletion loops formed during slippage at repetitive sequences. In humans, MutS homologs (MSH2/MSH6) recognize the mismatch; MutL homologs (MLH1/PMS2) coordinate excision and resynthesis. Loss of MMR yields microsatellite instability (MSI)—length variability in short tandem repeats observable on PCR assays. Tumors with MSI accumulate frameshift mutations in growth-regulatory genes, accelerating carcinogenesis. Lynch syndrome (hereditary nonpolyposis colorectal cancer, HNPCC) is the flagship MMR disorder. Germline pathogenic variants in MLH1, MSH2, MSH6, or PMS2 predispose to early colorectal cancer (often right-sided), endometrial carcinoma, and, variably, ovarian, gastric, urinary tract, pancreatic, and biliary malignancies. Unlike APC-driven familial adenomatous polyposis, Lynch does not require dozens to hundreds of adenomas; rather, fewer polyps progress more rapidly. The Muir–Torre variant features sebaceous neoplasms and keratoacanthomas alongside internal malignancies. On Step 1, recognition cues include early colon or endometrial cancer with a striking family history across generations and an “MSI-high” pathology report. Immunohistochemistry may show loss of MLH1 (often with PMS2) or MSH2 (with MSH6). MLH1 loss in sporadic tumors commonly reflects promoter hypermethylation rather than germline mutation—a frequent distractor. When repair genes are lost, frameshift-prone loci (e.g., TGF-β receptor II) become mutational hot spots, a concept testable in advanced stems. The clinical thread for examinees is surveillance and prevention logic: colonoscopy at earlier and more frequent intervals, consideration of endometrial cancer risk management, and cascade testing of relatives. Mechanistically, anchor “S-phase proofreading backup” to MMR, “MSI-high” to Lynch, and “sebaceous tumors” to Muir–Torre. These associations appear repeatedly and can be answered rapidly without full re-derivation if the pathway–phenotype map is fluent. 4) Mismatch Repair and Microsatellite Instability: Lynch and Its Variants
Double-strand breaks (DSBs) arise from ionizing radiation, topoisomerase poison exposure, collapsed replication forks, and interstrand crosslinks. Cells deploy two principal solutions. Homologous recombination (HR) uses the intact sister chromatid as a template to restore sequence faithfully—high fidelity but restricted to late S and G2. Non-homologous end joining (NHEJ) ligates broken ends directly—template-free and therefore error-prone—available throughout the cycle. Checkpoint kinases ATM and ATR detect DSBs and stalled forks, pausing the cycle and recruiting repair complexes; p53 integrates these signals to decide repair versus apoptosis. BRCA1/2 are core HR mediators; pathogenic variants compromise accurate DSB repair and elevate risks for breast, ovarian, and related cancers. Tumors lacking HR capacity exhibit “BRCAness” and dependency on alternative repair routes, a vulnerability exploited therapeutically with PARP inhibitors. Fanconi anemia (FA) is a multi-gene failure of interstrand crosslink repair that intersects HR; patients exhibit congenital anomalies (short stature, thumb/radial defects), progressive bone-marrow failure, and sensitivity to crosslinking agents. Laboratory testing shows chromosomal breakage after diepoxybutane or mitomycin C exposure. Ataxia-telangiectasia (ATM mutations) blends checkpoint failure with impaired DSB response. Clinical hallmarks are progressive cerebellar ataxia, oculocutaneous telangiectasias, immunodeficiency (impaired class switching), and marked radiosensitivity; alpha-fetoprotein tends to be elevated. LIG4 syndrome (DNA ligase IV deficiency) and defects in components such as Artemis (DCLRE1C) cause NHEJ failure with microcephaly, growth retardation, infection susceptibility, and radiosensitivity—SCID-like presentations that appear in pediatric stems. Bloom syndrome (BLM helicase) features genomic instability with high sister-chromatid exchange and proportionally increased risk of leukemias and solid tumors. Step 1 often cues Bloom with photosensitivity, proportional short stature, immunodeficiency, and a background of recurrent infections. Clinical management across these entities centers on avoidance of ionizing radiation, thoughtful use of DNA-damaging chemotherapy, infection prophylaxis where relevant, and cancer surveillance. Mechanistically, “HR → BRCA/FA,” “checkpoint signaling → ATM,” and “NHEJ core ligation → Ligase IV/Artemis” are the rapid-fire anchors to internalize. 5) Double-Strand Break Repair: HR, NHEJ, Checkpoints, and Clinically Salient Syndromes
Genomic imprinting is parent-specific epigenetic silencing—certain alleles are methylated and transcriptionally quiet depending on maternal or paternal origin. Disease results when the expressed allele is lost by deletion, mutation, or replaced by uniparental disomy (UPD). Two loci dominate medical testing. On chromosome 15q11-q13, paternal genes are normally expressed in certain neurons while the maternal copy is imprinted (silenced); loss of the paternal contribution yields Prader–Willi syndrome. Conversely, maternal UBE3A expression is critical in the brain, and loss of the maternal contribution yields Angelman syndrome. UPD comes in two flavors: heterodisomy (meiosis I error; two different homologs from one parent) and isodisomy (meiosis II error; two identical copies), the latter unmasking autosomal recessive mutations by duplicating a single parental allele. Laboratory confirmation often couples methylation-sensitive assays with copy-number testing and, when needed, trio genotyping to prove UPD. Step 1 typically requires only the parent-of-origin logic and signature phenotypes. Clinical implications include tumor surveillance in Beckwith–Wiedemann, nutrition and endocrine support in Prader–Willi, and seizure/rehabilitation strategies in Angelman. For test-taking efficiency, memorize: “Paternal missing → Prader–Willi; Maternal missing → Angelman,” and “11p15 imprinting → overgrowth and tumor risk.” 6) Genomic Imprinting and Uniparental Disomy: Parent-of-Origin Rules at the Bedside
Imprinting Disorder Genetic Mechanism Key Features Prader–Willi (15q11-q13) Paternal deletion or maternal UPD (two maternal copies) Neonatal hypotonia → hyperphagia/obesity, hypogonadism, short stature, learning issues Angelman (UBE3A on 15q11-q13) Maternal deletion/mutation or paternal UPD Seizures, ataxia, severe intellectual disability, frequent laughter/smiling Beckwith–Wiedemann (11p15) Imprinting defects/UPD with IGF2/H19 dysregulation Macroglossia, organomegaly, hemihyperplasia, omphalocele; Wilms/hepatoblastoma risk Russell–Silver Maternal imprinting disturbances (e.g., maternal UPD7) Intrauterine and postnatal growth restriction, triangular facies, limb asymmetry
Triplet-repeat disorders expand unstable short tandem repeats during gametogenesis, producing anticipation—worsening severity or earlier onset in successive generations. Expansion biases can be parent-specific: Huntington disease expansions are classically paternal; Fragile X expansions arise during oogenesis. Pathogenicity stems from different mechanisms: loss of function via hypermethylation and silencing (Fragile X), toxic gain-of-function proteins (Huntington), RNA splicing derangements (myotonic dystrophy type 1), or impaired mitochondrial function and iron handling (Friedreich ataxia). Step 1 vignettes emphasize life stage and systems clues: a teenage boy with developmental delay and macroorchidism after puberty (Fragile X), a 40-year-old with new-onset chorea and family history of early cognitive decline (Huntington), a young adult with handgrip myotonia and cataracts (DM1), or an adolescent with gait instability, loss of vibratory sense, and concentric left ventricular hypertrophy (Friedreich). Remember that premutation carriers in Fragile X can present with tremor/ataxia (FXTAS) or primary ovarian insufficiency—board-relevant but less emphasized. Quick mnemonics aid encoding: “Caudate loss in Huntington is CAG,” “Friedreich is Foot deformities and Failing heart,” and “CGG = Child with big Gonads and Giant ears.” Anchor these with mechanism verbs—silencing, toxic protein, toxic RNA—to keep look-alikes separate under time pressure. 7) Dynamic Mutations: Triplet-Repeat Expansion, Anticipation, and the “Big Four”
Disorder Repeat (Gene/Region) Mechanism Signature Features Fragile X syndrome CGG in FMR1 (5′ UTR) Hypermethylation → transcriptional silencing Intellectual disability, long face, large ears, macroorchidism; autism traits Huntington disease CAG in HTT (coding region) Toxic gain-of-function; neuronal loss (caudate) Chorea, psychiatric changes, dementia; paternal anticipation Myotonic dystrophy type 1 CTG in DMPK (3′ UTR) Toxic RNA → splicing defects Myotonia, distal weakness, cataracts, balding, testicular atrophy; congenital form via maternal transmission Friedreich ataxia GAA in FXN (intron) Reduced frataxin → mitochondrial dysfunction Progressive ataxia, loss of proprioception, pes cavus, scoliosis, hypertrophic cardiomyopathy, diabetes
Mini-vignettes. (A) A 6-year-old with severe sunburns after minimal sun, numerous lentigines, corneal clouding, and early skin cancers: defective NER → xeroderma pigmentosum. (B) A 23-year-old with hundreds of small adenomas is not required for early colon cancer; instead, a 44-year-old with few proximal polyps and MSI-high histology points to MMR loss → Lynch. (C) Growth retardation with triangular face and limb asymmetry suggests imprinting disturbance → Russell–Silver. (D) A 2-year-old with macroglossia, omphalocele, and hemihyperplasia needs tumor surveillance → Beckwith–Wiedemann. (E) A teen with myotonia and cataracts → CTG expansion (DM1). (F) A child with cerebellar ataxia, telangiectasias, recurrent sinopulmonary infections, elevated AFP → ATM deficiency. Common pitfalls. Confusing NER with photolyase (absent in humans); assuming all early colon cancers are APC-driven polyposis rather than MSI-high Lynch; forgetting that Angelman is maternal loss (UBE3A) and Prader–Willi is paternal loss at 15q11-q13; mixing HR (template-based, BRCA) with NHEJ (template-free, Ligase IV). Another frequent trap is treating Friedreich ataxia as a cortical process; it is a spinocerebellar-dorsal column disease with cardiomyopathy and diabetes risk. Exam execution. Lead with mechanism mapping, confirm with one discriminating clinical detail, and commit to an answer. These entities are engineered by test writers to be high-signal with short paths to recognition if the pathway–phenotype pairs are fluent. 8) Integration: Mini-Vignettes, Pitfalls, and a Rapid-Review Checklist
Rapid-Review Checklist
Board-Style Associations